Abstract
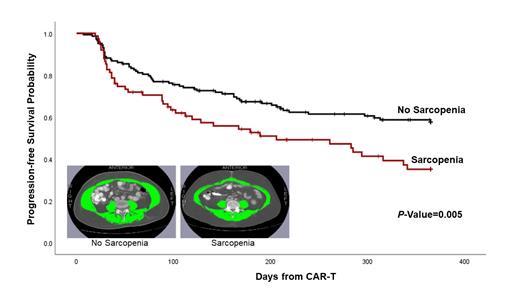
Introduction: Chimeric antigen receptor T cell (CAR-T) therapy is an effective treatment for patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL) or other B-cell lymphomas. However, the potent anti-lymphoma effect of CAR-T is balanced by the risk of acute toxicities, namely cytokine release syndrome (CRS) and Immune effector cell-Associated Neurotoxicity Syndrome (ICANS), as well as the variable length of progression-free survival (PFS) after CAR-T. Tools to better risk-stratify for adverse outcomes and to guide targeted interventions are lacking. Sarcopenia (loss of lean muscle mass) is an important cause of age-related functional decline in the general population and is an independent predictor of health outcomes in patients with solid and hematologic cancers, irrespective of age or comorbidity. Advances in software technology have facilitated the near real-time integration of body composition measurements into imaging studies obtained as part of standard clinical care. To date, there have been no studies to examine the association between sarcopenia and outcomes after CAR-T therapy. Methods: Using a retrospective cohort design, 280 consecutive patients with DLBCL or B-cell lymphoma, age ≥18y, and treated with CAR-T therapy between 2015 to 2020 at a single center were included in the study. This analysis was restricted to 226 (80.7%) patients with available computed tomography scans ≤60d from CAR-T. Skeletal muscle area was ascertained from abdominal scans using an automatic image analysis software (APACS; Voronoi Health Analytics; Vancouver, Canada); 3rd lumbar vertebra was used as a landmark because of its high correlation with whole-body muscle mass (J Clin Oncol 2016 34:1339); Figure. Trained researchers blinded to patient demographics and outcomes manually validated these measurements (SliceOmatic; Tomovision; Quebec, Canada). Skeletal muscle index (SMI) was calculated as the ratio of skeletal muscle area (cm 2) divided by height (m). Sarcopenia was defined according to sex-based cutoffs (lowest SMI tertile). Kaplan-Meier method was used to examine PFS at one-year. Multivariable regression was used to calculate the hazard ratio (HR) for PFS and odds ratio (OR) for toxicities with 95% confidence intervals (CI), adjusted for covariates (demographics [age, race/ethnicity], disease characteristics [largest lymph node diameter, blood lactate dehydrogenase], CAR-T product, ECOG performance status). Results: Median age at CAR-T was 63y (range: 18-84); 65.9% were male; 50.9% were non-Hispanic white; 8.8% had ECOG ≥2; 80.5% had a diagnosis of DLBCL; CAR-T products: axicabtagene ciloleucel (51.3%), lisocabtagene maraleucel (31.9%), other (16.8%); 46.9% were treated on a clinical trial; median residual lymph node diameter prior to CAR-T was 2.3cm (range: 0-17.2); 8.0% underwent HCT <1 year after CAR-T and follow-up was censored at HCT. Outcomes: 59.1% developed CRS (18.2% grade ≥2) and 30.1% developed ICANS (15.9% grade ≥2). In adjusted analyses, the odds of developing CRS or ICANS was 1.9-fold (CRS: 1.89 [95%CI: 1.02-3.5], ICANS: 1.93 [1.06-3.51]) higher among patients who were sarcopenic (reference: normal body composition). Average length of hospitalization was also longer (25.6d vs. 21.9d; p=0.037) among patients with sarcopenia. Survival: One-year PFS for the overall cohort was 50.1% (±4.2); PFS was significantly worse for patients who were sarcopenic compared to those with normal muscle mass (35.1% [±6.2] vs. 57.7% [±4.3], p=0.005; Figure). In adjusted analyses, sarcopenia was associated with inferior one-year PFS (HR=1.73 [CI: 1.12-2.68]) compared to those with normal muscle mass. Conclusion: Sarcopenia is an important and independent predictor of outcomes after CAR-T with potential downstream health-economic consequences, including increased burden of acute toxicities and prolonged length of hospitalization. Taken together, these data form the basis for real-time decision making prior to CAR-T (e.g. pre-habilitation, consideration of alternative treatments), or during/shortly after CAR-T (e.g. increased supportive care, rehabilitation), setting the stage for innovative strategies to improve outcomes after CAR-T therapy.
Artz: Radiology Partners: Other: Spouse has equity interest in Radiology Partners, a private radiology physician practice. Budde: Merck, Inc: Research Funding; Amgen: Research Funding; Astra Zeneca: Research Funding; Mustang Bio: Research Funding; Novartis: Consultancy; Gilead: Consultancy; Roche: Consultancy; Beigene: Consultancy. Herrera: Merck: Consultancy, Research Funding; Gilead Sciences: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Karyopharm: Consultancy; Kite, a Gilead Company: Research Funding; Seagen: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; Tubulis: Consultancy; Genentech: Consultancy, Research Funding. Popplewell: Novartis: Other: Travel; Pfizer: Other: Travel; Hoffman La Roche: Other: Food. Shouse: Kite Pharmaceuticals: Speakers Bureau; Beigene Pharmaceuticals: Honoraria. Siddiqi: Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Juno therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Other: DSM Member, Speakers Bureau; PCYC: Speakers Bureau; Jannsen: Speakers Bureau; Dava Oncology: Honoraria; ResearchToPractice: Honoraria. Forman: Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Allogene: Consultancy; Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal